Timeline
If you would like to see an event added to the timeline, please email us at info@resultsforinformedchoice.org.
-

July 2019 WHO Regional Stakeholder Meeting
WHO’s Regional Stakeholder Meeting will take place in Lusaka, Zambia, July 10-11, 2019.
-

July 2019 WHO Guideline Development Group Convening
The WHO’s Guideline Development Group will convene in Geneva, Switzerland, from July 29 to July 31, 2019.
-

June 2019 ECHO Trial Results Released
Results were published in a peer-reviewed article and announced live at the 9th SA AIDS Conference on June 13, 2019.
-

February 2019 WHO Stakeholders’ Meeting on Hormonal Contraception and HIV
WHO to hold key stakeholders' meeting on hormonal contraception and HIV.
-
February 2019 Civil Society Pre-Meeting Consultation
Civil Society Pre-Meeting Consultation to be held in Lusaka, Zambia.
-

January 2019 Results for Contraceptive Choice Stakeholder Consultation
Johns Hopkins CCP and AVAC hold Results for Contraceptive Choice Stakeholder Consultation in DC.
-
December 2018 ECHO Participant Follow-up Completed
In December 2018, the ECHO study team announced that October 31, 2018, marked the end of participant follow-up visits in the ECHO Study.
-
November 2018 Advocacy and Communication Landscaping
Anticipating the results of the ECHO trial in mid-2019, starting in November 2018 Johns Hopkins Center for Communication Programs (CCP) began carrying out a landscape assessment of advocacy and communication needs...
-
May 2018 Joint HIV Prevention and Family Planning Meeting
A one-day meeting of the family planning and HIV/AIDS communities to discuss aligning messages and communications strategies in advance of the release of the ECHO trial findings.
-

February 2018 Family Planning Global Handbook Released
The Global Handbook is considered an essential resource on contraceptive methods for healthcare professionals. In the 2018 edition, page 438 contains counseling tips for considering progestin-only injectables where HIV risk is high.
-

March 2017 WHO Reclassification
A WHO technical consultation concluded that the available evidence indicates an association between use of progestogen injectables and an increased risk of acquiring HIV.
-

December 2016 Systematic Review Published
The systematic review was updated and WHO convened a technical consultation with experts in the field in December 2016.
-

January 2016 Strategic Communication Framework Developed
This Strategic Communication Framework released in January 2016 is a tool to assist country stakeholders in the adaptation and dissemination of information pertaining to HC and HIV risk...
-
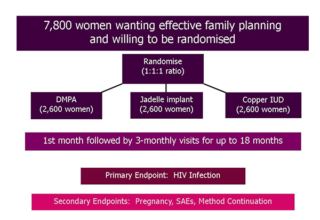
December 2015 ECHO Study Began
The ECHO study began in December 2015, when two sites in South Africa started screening and enrolling participants.
-

October 2015 Women Respond to WHO Statement
This statement rejects WHO’s October 21, 2015 “Statement on Depot-medroxyprogesterone acetate (DMPA)” and asks for a rigorous consultative process.
-

October 2015 Meeting Held on Developing Communication Strategies
HC3 hosted a meeting to provide the latest technical updates related to hormonal contraception and HIV...
-

October 2015 WHO Issues Statements on Reversible Hormonal Contraception Use
WHO issued two statements addressing concerns about the use of progestogen-only implants and depot-medroxyprogesterone acetate (DMPA).
-

July 2014 Medical Eligibility Criteria Category 1* Reaffirmed
WHO sponsored a systematic review to synthesize and evaluate the existing literature.
-
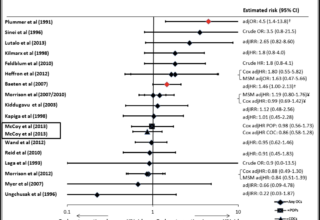
July 2014 Key Systematic Review Updated
This paper updated the July 2013 systematic review on hormonal contraception and HIV acquisition in women.
-

September 2013 PEPFAR and USAID Develop Technical Brief on Hormonal Contraception and HIV
PEPFAR and USAID jointly developed a brief to summarize the current epidemiological evidence regarding use of hormonal contraception and HIV acquisition.
-

July 2013 Systematic Review of Epidemiological Evidence Published
The systematic review of epidemiological data relating to HC and HIV acquisition included all relevant studies published (or in press) by December 15, 2011.
-

June 2012 CDC Analysis and Guidance Released
CDC assessed the evidence regarding hormonal contraceptive use and the risk for HIV acquisition, transmission, and disease progression.
-

February 2012 USAID Communication to the Field
USAID affirmed the WHO recommendations and, in a February 2012 communication to the field, stated that no changes should be made to country programs.
-

February 2012 WHO Convenes Technical Consultation
WHO convened a technical consultation from January 31 to February 1, 2012, to consider revising the guideline Medical eligibility criteria (MEC) for contraceptive use.
-

October 2011 USAID Communication to the Field on Heffron Study
USAID communication to the field on a study by Renee Heffron and colleagues that assessed the association between various methods of hormonal contraception and HIV risk.
-

October 2011 Heffron Study on Increased Risk Published
An October 2011 study found two-fold increases in the risks of both HIV acquisition and HIV transmission among women in HIV-discordant couples using hormonal contraceptives.
